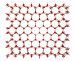
Ice Structures, Free Energies and Phase Diagrams
By comparing the free energies of the various polymorphs under common pressure and temperature conditions, we can understand which crystalline phase is thermodynamically preferred for particular water models and thus, which form we would expect it to adopt under unrestricted dynamics.
In order to perform this free energy comparison, the computational technique of thermodynamic integration was used on several different ice polymorphs. Thermodynamic integration is an established technique that has been successfully used in the past to evaluate the free energies of both liquid and solid phases of water. Both the liquid and solid systems are slowly transformed into systems where the partition function can be solved for analytically (an Einstein crystal for solids). We know the free energy at the transformed state and can determine the change in free energy during the transformation. Therefore, it is relatively easy to back out the free energy of the initial system. This process was used on five different low pressure ice polymorphs (ice Ih, ice Ic, ice B, Ice-i, and Ice-i´) using six different common water models (TIP3P, TIP4P, TIP5P, SPC/E, SSD/E, and SSD/RF). Results for the 9 Å cutoff simulations are shown in the following table:
Click on the crystal images for a more detailed view.
In all of these simulations, Ice-i (or Ice-i´) has the lowest free energy for all of the water models studied. Another interesting point is the fact that the melting temperatures (with the obvious exception of SSD/E) show reasonably good agreement with the experimental value of 273 K, i.e. much better than the values observed for TIP4P from ice Ih (from 214 to 238 K). An important distinction to note is that these melting transitions are calculated from the most stable phase (Ice-i or Ice-i´ in this case), and calculation of the Tm from ice Ih from this work places TIP4P’s melting transition at ~210 K – in line with the estimates from other groups.